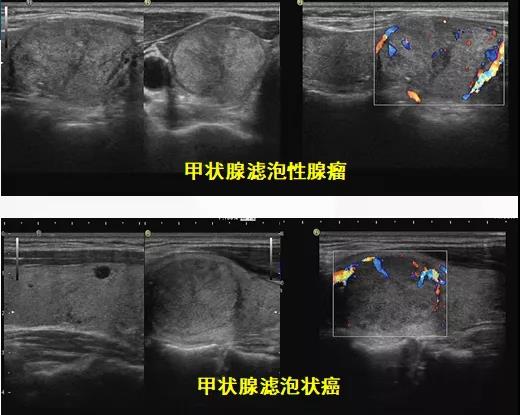
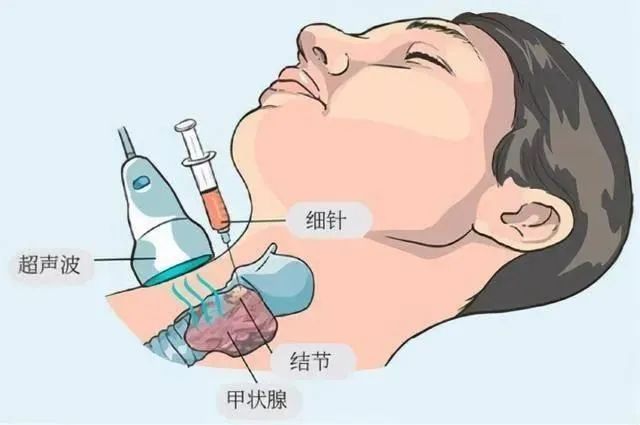
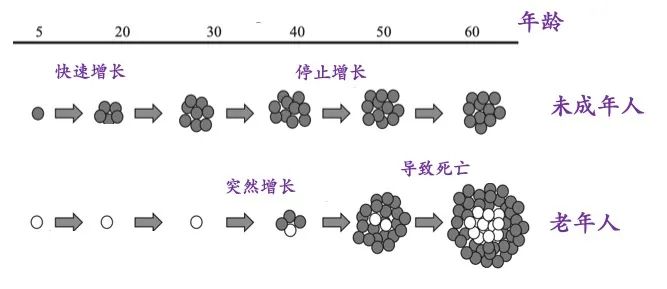
参考文献L93帝国网站管理系统
(在框内滑动手指即可浏览)L93帝国网站管理系统
L93帝国网站管理系统
[1] Lim H,Devesa SS,Sosa JA,et al.Trends in thyroid cancer incidence and mortality in the United States, 1974-2013[J].JAMA,2017,317(13):1338-1348.L93帝国网站管理系统
[2] 郑荣寿,陈茹,韩冰峰,等.2022年中国恶性肿瘤流行情况分析[J]. 中华肿瘤杂志,2024,46(3):221-231.L93帝国网站管理系统
[3] Surveillance Research Program. SEER incidence data,November 2022 submission(1975-2020),SEER 22 registries(excluding Illinois and Massachusetts)[DB/OL]. National Cancer Institute. Updated June 8,2023.Accessed September 17,2023. https://seer.cancer.gov/statistics-network/explorer/application.html.L93帝国网站管理系统
[4] Baloch ZW,Asa SL,Barletta JA,et al.Overview of the 2022 WHO classification of thyroid neoplasms[J].Endocr Pathol,2022,33(1):27-63.L93帝国网站管理系统
[5] Xu B,David J,Dogan S,et al.Primary high-grade non-anaplastic thyroid carcinoma: a retrospective study of 364 cases[J].Histopathology,2022,80(2):322-337.L93帝国网站管理系统
[6] Wong KS,Dong F,Telatar M,et al.Papillary thyroid carcinoma with high-grade features versus poorly differentiated thyroid carcinoma: an analysis of clinicopathologic and molecular features and outcome[J].Thyroid,2021,31(6):933-940.L93帝国网站管理系统
[7] Wendler J,Kroiss M,Gast K,et al.Clinical presentation, treatment and outcome of anaplastic thyroid carcinoma: results of a multicenter study in Germany[J].Eur J Endocrinol,2016,175(6):521-529.L93帝国网站管理系统
[8] Bonhomme B,Godbert Y,Perot G,et al.Molecular pathology of anaplastic thyroid carcinomas: a retrospective study of 144 cases[J].Thyroid,2017,27(5):682-692.L93帝国网站管理系统
[9] Landa I,Ibrahimpasic T,Boucai L,et al.Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers[J].J Clin Invest,2016,126(3):1052-1066.L93帝国网站管理系统
[10] 中国医师协会外科医师分会甲状腺外科医师委员会,中国抗癌协会甲状腺癌专业委员会,中国研究型医院学会甲状腺疾病专业委员会.甲状腺髓样癌诊断与治疗中国专家共识(2020版)[J]. 中国实用外科杂志,2020,40(9):1012-1020.L93帝国网站管理系统
[11] Gu H,Wang J,Ran W,et al.Anaplastic and poorly differentiated thyroid carcinomas: genetic evidence of high-grade transformation from differentiated thyroid carcinoma[J].J Pathol Clin Res,2024,10(2):e356.L93帝国网站管理系统
[12] Nguyen B,Fong C,Luthra A,et al.Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients[J].Cell,2022,185(3):563-575.L93帝国网站管理系统
[13] Toraih EA,Hussein MH,Zerfaoui M,et al.Site-specific metastasis and survival in papillary thyroid cancer: the importance of brain and multi-organ disease[J].Cancers (Basel),2021,13(7):1625.L93帝国网站管理系统
[14] Jr SD,Ho A,Chintakuntlawar AV,et al.American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: Defining advanced thyroid cancer and its targeted treatment[J].Head Neck,2022,44(6):1277-1300.L93帝国网站管理系统
[15] Brose MS,Nutting CM,Jarzab B,et al.Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial[J].Lancet,2014,384(9940):319-328.L93帝国网站管理系统
[16] Schlumberger M,Tahara M,Wirth LJ,et al.lenvatinib versus placebo in radioiodine-refractory thyroid cancer[J].N Engl J Med,2015,372(7):621-630.L93帝国网站管理系统
[17] Schlumberger M,Elisei R,Müller S,et al.Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma[J].Ann Oncol,2017,28(11):2813-2819.L93帝国网站管理系统
[18] Brose MS,Robinson B,Sherman SI,et al.Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial[J].Lancet Oncol,2021,22(8):1126-1138.L93帝国网站管理系统
[19] Jr WS,Robinson BG,Gagel RF,et al.Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial[J].J Clin Oncol,2012,30(2):134-141.L93帝国网站管理系统
[20] Subbiah V,Hu MI,Wirth LJ,et al.Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study[J].Lancet Diabetes Endocrinol,2021,9(8):491-501.L93帝国网站管理系统
[21] Hadoux J,Elisei R,Brose MS,et al.Phase 3 trial of selpercatinib in advanced RET -mutant medullary thyroid cancer[J].N Engl J Med,2023,389(20):1851-1861.L93帝国网站管理系统
[22] Wirth LJ,Sherman E,Robinson B,et al.Efficacy of selpercatinib in RET -altered thyroid cancers[J].N Engl J Med,2020,383(9):825-835.L93帝国网站管理系统
[23] Subbiah V,Kreitman RJ,Wainberg ZA,et al.Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study[J].Ann Oncol,2022,33(4):406-415.L93帝国网站管理系统
[24] Busaidy NL,Konda B,Wei L,et al.Dabrafenib versus dabrafenib + trametinib in BRAF -mutated radioactive iodine refractory differentiated thyroid cancer: results of a randomized, phase 2, open-label multicenter trial[J].Thyroid,2022,32(10):1184-1192.L93帝国网站管理系统
[25] Waguespack SG,Drilon A,Lin JJ,et al.Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma[J].Eur J Endocrinol,2022,186(6):631-643.L93帝国网站管理系统
[26] Demetri GD,De Braud F,Drilon A,et al.Correction: updated integrated analysis of the efficacy and safety of entrectinib in patients with NTRK fusion-positive solid tumors[J].Clin Cancer Res,2022,28(10):2196.L93帝国网站管理系统
[27] Li D,Chi Y,Chen X,et al.Anlotinib in locally advanced or metastatic medullary thyroid carcinoma: a randomized, double-blind phase IIB trial[J].Clin Cancer Res,2021,27(13):3567-3575.L93帝国网站管理系统
[28] Chi Y,Zheng X,Zhang Y,et al.Anlotinib in locally advanced or metastatic radioiodine-refractory differentiated thyroid carcinoma: a randomized, double-blind, multicenter phase II trial[J].Clin Cancer Res,2023,29(20):4047-4056.L93帝国网站管理系统
[29] Lin Y,Qin S,Yang H,et al.Multicenter randomized double-blind phase III trial of donafenib in progressive radioactive iodine-refractory differentiated thyroid cancer[J].Clin Cancer Res,2023,29(15):2791-2799.L93帝国网站管理系统
[30] Fugazzola L,Elisei R,Fuhrer D,et al.2019 European Thyroid Association Guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer[J].Eur Thyroid J,2019,8(5):227-245.L93帝国网站管理系统
[31] Wirth LJ,Durante C,Topliss DJ,et al.lenvatinib for the treatment of radioiodine-refractory differentiated thyroid cancer: treatment optimization for maximum clinical benefit[J].Oncologist,2022,27(7):565-572.L93帝国网站管理系统
[32] Leboulleux S,Do Cao C,Zerdoud S, et al. A Phase II redifferentiation trial with dabrafenib-trametinib and 131I in metastatic radioactive iodine refractory BRAFV600E-Mutated differentiated thyroid cancer[J].Clin Cancer Res,2023,29(13):2401-2409.L93帝国网站管理系统
[33] Chintakuntlawar AV,Rumilla KM,Smith CY,et al. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study[J]. J Clin Endocrinol Metab,2017,102(6):1943-1950.L93帝国网站管理系统
[34] Dierks C,Seufert J,Aumann K,et al.Combination of lenvatinib and pembrolizumab is an effective treatment option for anaplastic and poorly differentiated thyroid carcinoma[J].Thyroid,2021,31(7):1076-1085.L93帝国网站管理系统
[35] Ji DM,Shen WN,Kuang MY,et al. A phase II study to evaluate the efficacy and safety of Camrelizumab plus famitinib in advanced or metastatic thyroid cancer[J]. J Clin Oncol,2022,40(16):6085.L93帝国网站管理系统
[36] Wang JR,Zafereo ME,Dadu R,et al.Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAFV600E -mutated anaplastic thyroid carcinoma[J].Thyroid,2019,29(8):1036-1043.L93帝国网站管理系统
[37] Chen JY,Huang NS,Wei WJ,et al.The efficacy and safety of surufatinib combined with anti pd-1 antibody toripalimab in neoadjuvant treatment of locally advanced differentiated thyroid cancer: a phase II study[J].Ann Surg Oncol,2023,30(12):7172-7180.L93帝国网站管理系统
[38] Chen J,Ji Q,Bai C,et al.Surufatinib in Chinese patients with locally advanced or metastatic differentiated thyroid cancer and medullary thyroid cancer: a multicenter, open-label, phase II trial[J].Thyroid,2020,30(9):1245-1253.L93帝国网站管理系统